How to Find Number of Electrons
Repeat for each element in the molecule then sum together all the products to get the total amount of electrons. How to Determine the Number of Excess Electrons on an Object Given its Net Charge in Coulombs.

3 Ways To Calculate Atomic Mass Wikihow Teaching Chemistry Atoms And Molecules For Kids Chemistry Worksheets
Step 4 Find the number of valence electrons by counting the total number of electrons present in the outermost shell.

. Carbon-14 has two additional neutrons but the same number of protons and elec. It is not the same as the atomic weight. To convert from charge per second to number of electrons we will divide by the fundamental unit of charge and multiply by.
Identify the proton number also called atomic number of the element on the periodic table of elements. It resides in a specific orbit of the atom and revolves around the nucleus. This is a chemistry tutorial video that goes through how to find the number of electrons in each energy level or each electron shell including how to find t.
Calculate the number of electrons for the given time interval. The number of electrons in KNO3 in the first case is equal to 19 x 1 7 x 1 8 x 3 50. 11 atomic mass 5 atomic number 6 neutrons.
Answer 1 of 3. Determine the number of Coulombs of electrons passing through the wire over a single second. Lets take one more example to understand How to calculate the valence electron with the help of.
Number of protons present in an atom. Calculate the number of protons neutrons and electrons it contains. Now express the.
The outermost shell of nitrogen has 2s 2 2p 3 therefore the total valence electrons in nitrogen are 2 3 5. How to find the Atomic Number. A pure substance that cannot be broken down into a simpler substance by chemical means.
The mass number of the atom M is equal to the sum of the number of protons and neutrons in the nucleus. Determine the net charge eqQ eq on the object in Coulombs. How to Determine the Number of Electrons from the Proton Number.
For example the most common isotope of carbon carbon-12 has 6 protons 6 electrons and 6 neutrons. The properties of the elements and their compounds depend on the electron configuration. Thomson discovered the existence of electrons.
The atomic number of a sodium atom is 11 and its mass number is 23. In 1897 scientist J. Multiply the elements atomic number by the number of atoms of this kind in the molecule see Step 1.
How to find the number of electrons in an element. The nuclear number of an component is simply the total of protons in. There are 0009 Coulombs that pass through.
Electrons are the permanent core particles of an atom. The number of electrons is the same as the atomic number. A weighted average of the number of neutrons and protons present for all isotopes.
For example boron B has an atomic number of 5 therefore it has 5 protons and 5 electrons.

Finding Protons Neutrons And Electrons Through The Atomic Number And Neutrons By Mass Atomic Proton Neutron Electron Protons Neutrons
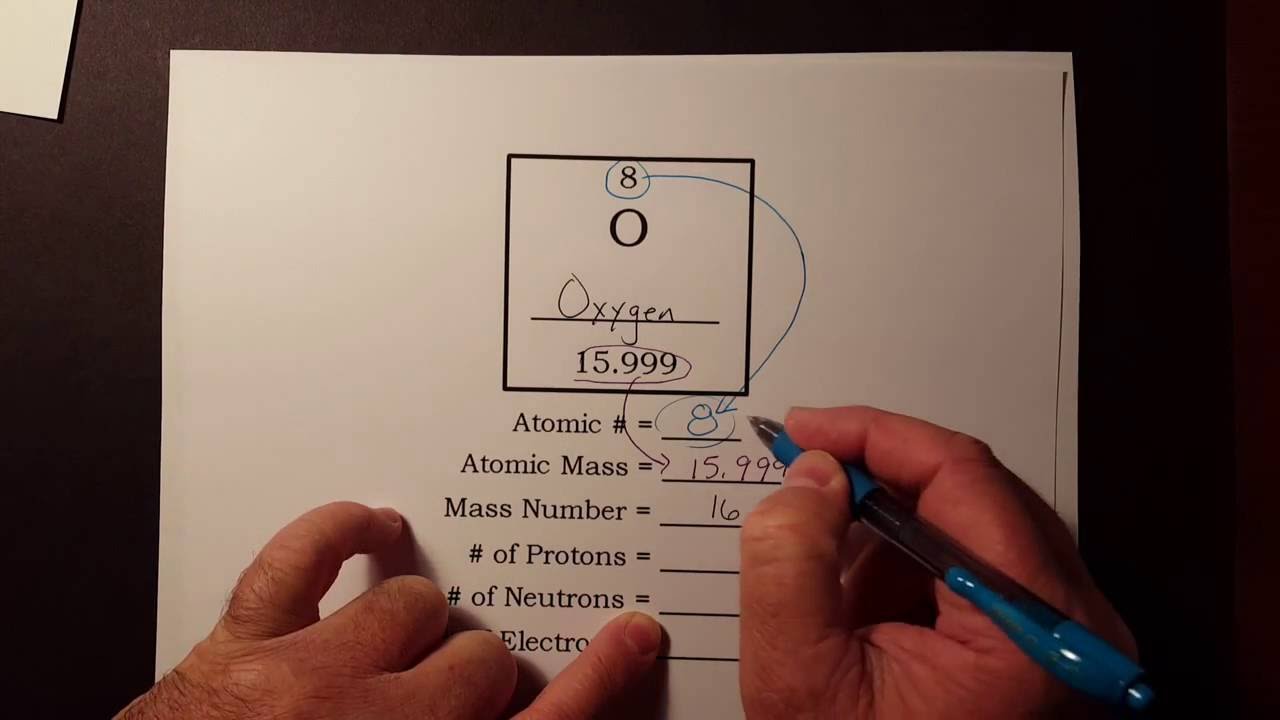
How To Find The Number Of Protons Neutrons And Electrons From The Periodic Table Youtube Neutrons Protons Proton Neutron Electron

Comments
Post a Comment